IONIC
COVALENT
METALLIC
VAN DER WAALS FORCES
UNIT CELL
LATTICE STRUCTURE
BCC
FCC
HCP
DISLOCATIONS
VACANCIES
INTERSTITIAL
GRAIN SIZE
GRAIN BOUNDARIES
SLIP
ALLOTROPIC
RECRYSTALLIZATION
INTRODUCTION: In Module 1 we
looked primarily at some mechanical properties of materials including the
plastic deformation of materials, the relationship between stress and strain,
and external loading. In Module 2, we will begin looking at the atomic
structures of materials and what makes materials behave the way they do,
why some are better conductors of heat than others, why some are brittle
while others are ductile, and so forth.
This module will focus mostly on the metals
group. Polymers will be covered in Module 4.
There are literally thousands of materials available however they can be divided into 3 major categories:
METALLIC
CERAMIC
POLYMERIC (INCLUDING WOOD)
These groupings are made because of the bonding forces (which depend on the atomic arrangement) are similar within a group. As we look deeper into the bonding forces, the reasons for varying properties such as harness, toughness, conductivity and strength can be more easily understood. First we need to review some basic atomic theory.
ATOMIC THEORY
The nucleus of the atom is made up of positively charged protons and neutrally charged neutrons. The nucleus makes up most of the weight of the atom, and elements can can be categorized by atomic weight. However, Atomic number determines the properties and characteristics of materials. So, what is the atomic number? It is simply the number of protons in the nucleus. The diagram below shows a simple model. Notice that the nucleus is positively charged and made up of "big protons and neutrons". But, this nucleus is surrounded by electrons that are negatively charged. An atom in the free state must be electronically neutral. Thus, the number of electrons surrounding the nucleus must equal the number of protons in the nucleus...this is the atomic number.
The number, arrangement, and spin of the electrons determines the kind of atom and its characteristics such as crystal forms, melting points. When shells are filled, the atom is stable; however electrons that are in unfilled shells are know as valence electrons and are largely responsible for the behavior of the element. Partially filled shells may give up or receive (accept extra electrons) or may share them with other atoms. The manner in which this "stabilization is reached determines the type of bonding. The period table arranges the elements in rows numbered from 1 to 103. Why this number? What is it? The atomic number is simply the stabilized number of electrons and protons. For example, Hydrogen (H) has one proton and one electron; Helium (He) has two electrons and 2 protons....all the way up to Lawrencium (LR) which has 103 electrons and protons. The periodic table in your text also shows classifications of metals by characteristics. In a complete period table, the atomic weight is also shown for each element.
NOTE: REFER TO YOUR TEXTBOOK ON PAGE 18
OR CLICK HERE TO GO TO THE
ONLINE
PERIOD TABLE.
.
As mentioned previously, materials are typically divided into 3 major simple classes: metallic; ceramic; and polymeric. These groupings are made because of the bonding forces which depend on the atomic arrangement are similar within a group. As we look deeper into the bonding forces, an understanding of the reasons for varying properties such as hardness, toughness, conductivity, and strength can be seen.
BONDING: When shells are filled, the atom is stable; however, electrons that are in unfilled shells are know as valence electrons. These valence electrons are largely responsible for the behavior of the element. It should be noted that metals are characterized by an ability to give up electrons. Partially filled shells mean that electrons may be given up, accepted from other atoms, or may share them with other atoms. The manner in which this "stabilization is acquired determines the type of bonding. There are four types of bonding:
* IONIC
* COVALENT
* METALLIC
* VAN DER WAALS FORCES
IONIC: When an element gives up or receives an electron in its outer shell an ION is formed. If the element gives up an electron, it is then left with at net + 1 charge, and is called a POSITIVE ION. An example of ionic bonding can be seen between lithium and fluorine:
Ionic bonding accounts for many common liquids and solids such as NACL, AgCL, MgO.
Characteristics of materials with ionic bonding include:
Medium - High Strength
Hard but Brittle
High Melting
Electrically Insulating (because all charge "transport" must occur through
ION movement
COVALENT BONDING: This type of bonding occurs from sharing of valence electrons (electrons in the outer shell). Remember that the nuclei is POSITIVE (+), therefore, if electrons (-) are shared by adjacent nuclei, the result is a VERY strong bond.
Characteristics of materials having a covalent bond include:
High Strength
High Melting Point
Brittle
Electrical Conductivity Depends on Bond Strength (e.g. tin to diamond)
METALLIC BOND: A common characteristic of metallic elements is that they contain only 1, 2, or 3 electrons in their outer shell. Since there are fewer electrons, the bond is relative loose to the nucleus. When the outer shell (or valence) electrons approach each other's adjacent orbit, the electrons may be "forced out of natural orbit". This results in positive ions being formed. These floating electrons form a "cloud" of shared valence electrons, and electron movement can occur freely. This is why electrical and thermal conductivity is high in metals. However, metals crystallize into close paced lattices. We will discuss lattice structures in more detail later in the module. Metals make up over three fourths of the elements and have the following common characteristics
Metals make up over three fourths of the elements and have the following common characteristics:
Ability to form positive ions through donation of electrons;
Have a crystalline as opposed to amorphic structure;
Have excellent thermal and electrical conduction;
Have varying degrees of luster and reflectivity.
VAN DER WAAL BONDS (FORCES):
This bonds are weak bonds are found in neutral atoms such as inert gases.
Molecules are linked together through weak positive and negative attraction.
some parts of the molecules are more positive than others. This type
of bonding is of little interest in metals except for very low temperatures.
However; it is much more important in polymers.
ATOMIC PATTERN ARRANGEMENT: Rather than describing the billions of particles that make up the grain structure, it is easier to look at the basic "building blocks" or atomic arrangement that occurs in materials. In metals this basic building block is called the unit cell. In polymers it is referred to as a "mer". This module will focus on metals.
When metals change from a liquid to a solid,
grains are formed. This is referred to as grain growth.
The process begins with the atoms arranging themselves into geometric patterns.
There are six patterns that are formed in different types of metals:
Face Centered Cubic (FCC)
Body Centered Cubic (BCC)
Hexagonal close packed (HCP
Cubic
Body Centered tetragonal
Rhombohedral
The most common are the FCC, BCC and HCP. A two dimensional representation of this three types is show below.
Iron has a unique characteristic in its ability to change from BCC at room temperature to FCC at elevated temperatures and back to BCC at critically high temperatures. This ability to change states is referred to as allotropic. Refer to your textbook, page 21, figure 1.11.
Steel is more easily deformed at elevated temperatures because it changes from a BCC to a FCC structure. Lets look more closely at the general characteristics associated with BCC, FCC, and HCP structures.
BCC - Body centered cubic structures, are more difficult to deform due to the additional atom being present at the center of the structure. However, it is a catch-22.....also more slip planes are formed.
FCC - Face centered cubic structures are generally more easily deformed simply because there is no common atom in the center of the cubic structure. As grains grow, more slip planes are created. However, considerable force is still required to cause deformation. This is really what occurs when steel reaches its yield point.
HCP - This structure shape has fewer planes for orientation and slippage however, materials having this atomic arrangement tend to be more brittle.
Crystals (grains) depend on:
1. Lattice type
2. Inter atomic forces
3. Spacing between atoms
4. Density of atoms themselves
NOTE: During elastic deformation, the lattice structure
is shifted, stretched, and distorted, BUT RETURNS to the materials original
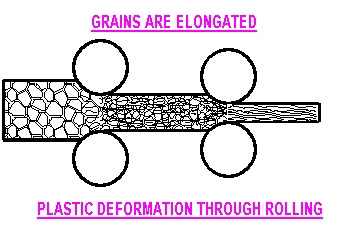
form. With plastic deformation, atoms change positions
and slip past one another because the atomic bond is broken.
Strength of a material also depends on crystal imperfections, alloying agents, and impurities. The diagram shown below shows examples of these factors.
There are 3 major classes of materials
Metals
Ceramic
Polymeric
Four types of atomic bonding exists
IONIC
COVALENT
METALLIC
VAN DER WAALS FORCES
Metals are made up of three basic crystal structures (UNIT CELL) types
BCC
FCC
HCP
Crystals grow to form grains, grains interfere during growth to form
boundaries.
Crystals are not perfect
-- have imperfections
Dislocations
Vacancies
Impurities
Inclusions
Interstitials
Material Properties depend on
Crystal
lattice
Grains are distorted
by boundaries.
Grain size is affected by temperature
Nature of defects
Some materials
have the ability to go through and allotropic
change
Iron (the base element in steel) is one of those materials.
Deformation may be elastic or plastic
Elastic deformation
- lattice structure is distorted but reorients
Plastic Deformation
occurs by slip along lattice, atomic bonds
are broken
Hardening may occur during plastic deformation
Results from entanglement of dislocations and grain boundaries
Can be eliminated by recrystallization at
elevated temperatures.
NOTE: USE OF HEAT TO ACHIEVE DESIRED PROPERTIES WILL BE COVERED
IN MODULE 3.
2. Would covalent bonded materials make good heating elements? Explain.
3. Draw and label an atomic model for tin showing the number of electrons in each shell.
4. How many valence electrons are there in problem 3?
5. Draw a diagram showing the phases of transition from liquid to solidification
in iron..from unit cell to grain boundary
formation.
6. Briefly discuss how current "flows" through a piece of copper wire.
7. Why is steel stronger at room temperature as opposed to elevated
temperatures above 1700 deg. F ? Hint:
Include an explanation of the term allotropic
in your response.
8. Of the 100 + elements, which are classified as heavy, brittle
metals? Hint: Locate a periodic table that breaks
down the classes of the elements. You
might want to consider the web for this.
9. Briefly explain how plastic deformation occurs in metals.
10. Explain the probable deformation characteristics of magnesium based
on its crystalline structure.